Our lives are made up of simple activities that help us thrive, be social, maintain…
THE FUTURE OF TMS: AN UPDATE
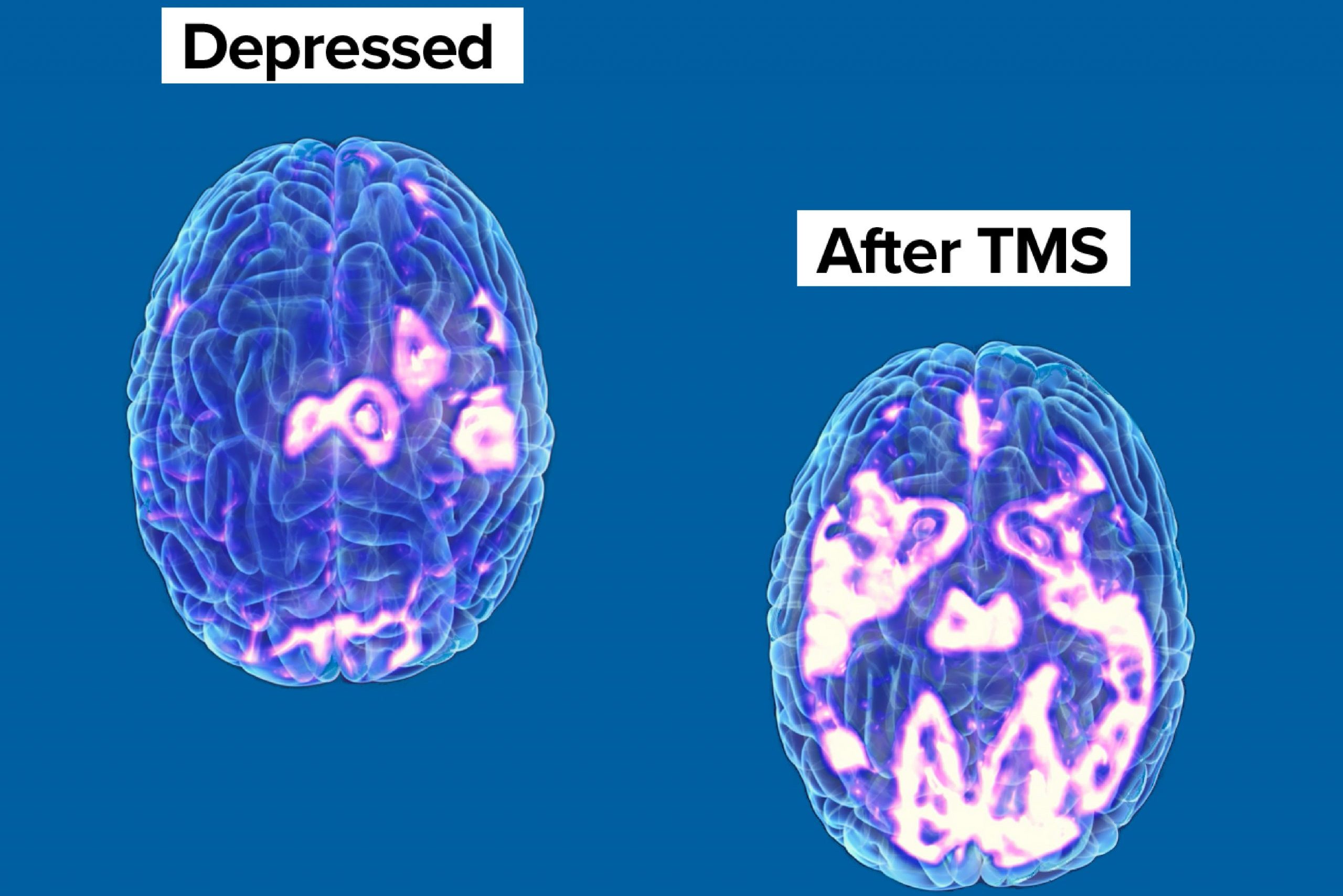
For decades, Transcranial Magnetic Stimulation (TMS) has been recognized worldwide as a non-invasive procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of depression and other neurological issues. As with any treatment, research is ongoing into the techniques and applications that will allow TMS to continue to expand its reach as an impactful and widely accepted treatment option.
In this blog, we provide an update on some of the recent studies that look toward the future of TMS and explore its efficacy in a variety of areas, including depression, pain, and post-acute motor stroke.
An Individualized Treatment
As Philip et al. note in The Future of Precision Transcranial Magnetic Stimulation in Psychiatry: “Researchers have been investigating various neuroimaging approaches to individualize TMS in order to match patients with the appropriate treatments.” These efforts have generally focused on 3 key outcomes:
- Understand more fully the relationship between specific stimulation sites in the brain and the neurological issue being treated; that is, how to target TMS more effectively.
- The specific parameters for stimulation that aligns with both the target site and the issue being treated.
- More accurate predictability of the treatment response.
Interestingly, these neuroimaging studies focus on two distinct strategies. The first and most obvious is to emphasize navigation to a site specifically correlated to the patient’s issue while considering differences in such anatomical features as head size and even variations in skull thickness. Known as scalp-based approaches, they provide clinicians with a set of variables that allow for individualized adjustments for both location and stimulation intensity.
The second strategy is to identify regions of the brain that are negatively correlated with the patient’s issue to use that target as the receptor of stimulation. For instance, imagine that a clinician is treating a patient for an issue that causes a certain area of the brain to become overactive or hyperactive (such as addiction). She can choose to stimulate the overactive area of the brain (as laid out above) or stimulate another area of the brain that is known to decrease neurological hyperactivity across the brain. By stimulating this anticorrelated area, the clinician effectively slows down activity in the area of the brain driving the addiction and, theoretically at least, begins to reduce at least one of the key neurological drivers of the addiction itself.
A third option, of course, is to combine stimulation of both correlated and anticorrelated targets, which is an option that is gaining moderate traction among researchers.
Several studies have suggested that this type of targeted simulation helps clinicians discover deep patterns of connectivity in a patient’s brain. This knowledge in turn feeds into complex predictive models that can better help clinicians understand the response and/or range of responses as well as predictors of both efficacy and potential remission rates. Because TMS is currently considered a limited resource in many locations, the ability to predict effectiveness will help clinicians make counsel individuals to the technology and treatment package that will deliver the best results over the shortest period of time as cost-effectively as possible.
Known as a decision curve analysis, this emerging technique is a relatively simple method for evaluating prediction models, diagnostic tests, and known neurological markers that allow clinicians to make more informed evidence-based, and in this case mathematically driven treatment decisions. The analysis is based on the assumption that with every treatment comes 4 potential outcomes: positive, negative, false positive, and false negative. Put simply, a decision curve analysis increases the likelihood of an optimized treatment plan for every patient while potentially reducing treatment times and costs. As Philip et al. explain:
“If we assume the average cost and time of a TMS treatment series to be $15,000 and 36 clinical visits, the currently used strategy results in the cost and time for each remission being $50,000 and 120 clinical visits. Use of theoretical predictive neuroimaging would reduce the costs and time for each remission to $37,142 and 77 clinical visits, even after accounting for the addition of a clinical visit and a $1,500 MRI scan for each patient.” (All figures have been chosen as broad, general estimates and are used for example purposes only.)
Conclusion
Though these evidence-based models have yet to be adopted as clinical best practices within TMS centers, there are obvious practical and economic reasons that they will transition from studies to clinics in the not-too-distant future. In fact, TMS might become the first neurological treatment option to engage neuroimaging as a guide to clinical practice.
Regardless, there is a tremendous potential for TMS clinicians to leverage emerging knowledge and incorporate new modeling techniques to make TMS more available and more effective for current and future generations of patients.